

PGT-M, or preimplantation genetic testing for monogenic diseases, is a genetic test developed to reduce the risk of having a child with an inherited genetic condition such as Sickle cell anemia, Fragile X syndrome, Muscular Dystrophy, and many others. It is performed prior to pregnancy by testing embryos during an in-vitro fertilization (IVF) cycle, before implantation, to ensure that only unaffected embryos are transferred back to the uterus.
Monogenic disorders result from mutations in a single gene. These conditions may have autosomal recessive, autosomal dominant, or X-linked inheritance patterns. PGT-M can be offered for most single-gene mutations if the specific gene mutation(s) in the family has been confirmed by DNA testing.
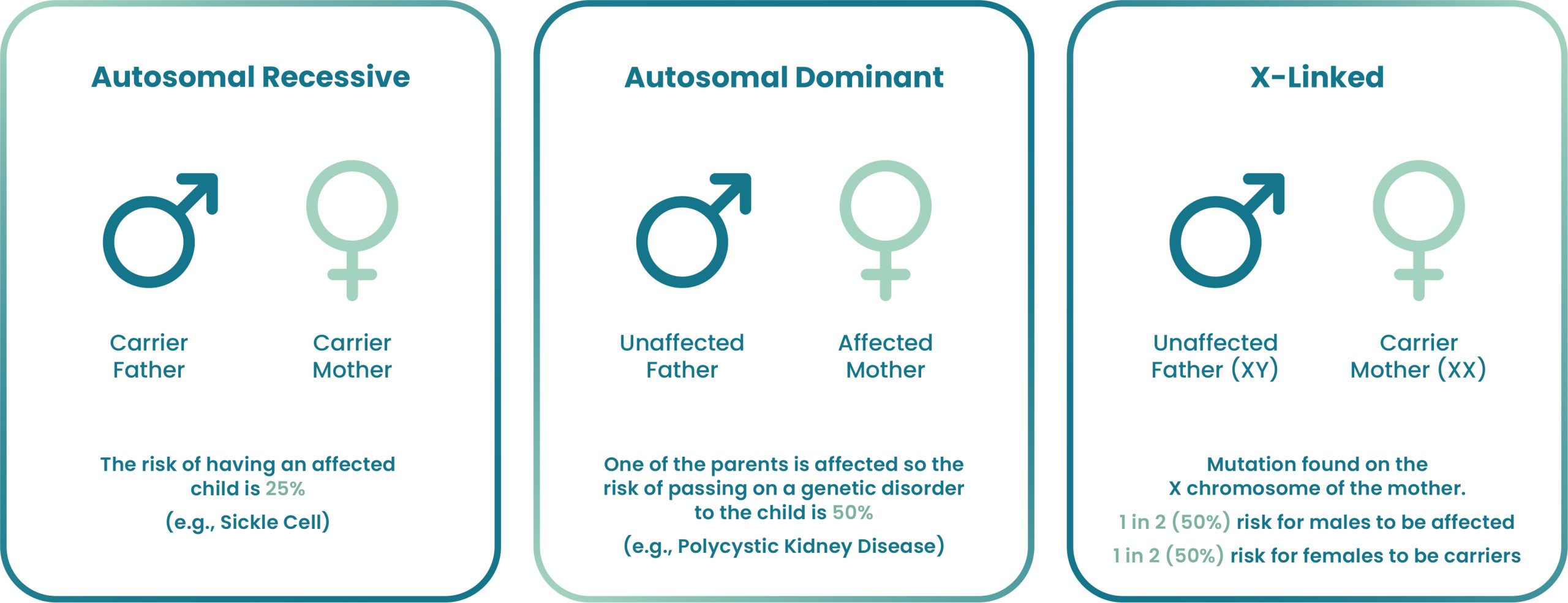

PGT-M is considered for couples who are at an increased risk of passing on a specific genetic condition. The test is beneficial when:
Each PGT-M test is customized for each family, so DNA samples from the carrier couple, and often additional family members are requested for the test to be designed & optimized. Only when First Genomix issues a confirmation for test completion, the couple can start their IVF treatment.
The PGT-M test involves a close examination of the mutation by sequencing or fragment analysis (based on the mutation type) along with haplotyping by linkage analysis. Through this approach, the gene mutation that causes the genetic disease in the family will be detected.
Linkage analysis is used to determine the area around the gene (haplotype) where genetic changes are analyzed to diagnose/classify each tested embryo as affected, unaffected, and/or carrier for the mutation.
The method used by First Genomix results in a more accurate screening process in terms of detecting carrier embryos and excluding contamination, as illustrated:


The PGT-M test is performed using sequencing and/or fragment analysis and linkage, with an accuracy rate of 99%. However, invasive prenatal testing is highly recommended to confirm that the fetus is unaffected. For the past 3 years, First Genomix has performed PGT-M for almost 500 different genes, with the most common being: HBB, SMN1, CFTR, FMR1, NF1, F8, PKHD1, TBCE.
Couples facing the risk of sporadic chromosome abnormalities in embryos have the option to undergo additional testing called Pre-implantation Genetic Testing for Aneuploidy (PGT-A) which helps in selecting the embryo with the highest chance of successful implantation. PGT-A can be performed alongside other tests on the same embryo biopsy, either on day 3 or day 5 of development. The inclusion of PGT-A in an IVF cycle does not alter the overall process or the time it takes to obtain results.
First Genomix offers rapid reporting, enabling embryos to be transferred either fresh or frozen, according to the IVF physician's protocol. Results for fresh PGT-M cases (testing for specific genetic mutations) are available within 36 hours of sample receipt at First Genomix, while frozen cases receive results within 72 hours.